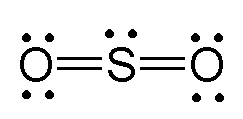Molecular Shape Of So2, Sulfur Dioxide So2 Molecule Model And Chemical Formula Stock Illustration Download Image Now Istock
Molecular shape of so2 Indeed lately is being sought by users around us, maybe one of you. Individuals now are accustomed to using the net in gadgets to view image and video data for inspiration, and according to the name of the article I will discuss about Molecular Shape Of So2.
- Solved Using Vsepr Theory Determine The Electron Group G Chegg Com
- Media Portfolio
- Vsepr Theory Part Ii Janet Gray Coonce
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcqtg8iahpgqar Uxidcjifad Yn7xuqc2my5zzl6qwchywtutbi Usqp Cau
- Why So Math 2 Math Has Bent Structure While Co Math 2 Math Is Linear Quora
- Chapter 6 Molecular Structure
Find, Read, And Discover Molecular Shape Of So2, Such Us:
- U World Gen Chem Bonding 1 Flashcards Quizlet
- The Shapes Of Molecules
- Solved For Each Of The Following Molecules Or Ions Draw Chegg Com
- So2 Lewis Structure How To Draw The Lewis Structure For So2 Sulfur Dioxide Youtube
- Chemical Bonding Ii Molecular Geometry And Hybridization Of Atomic Orbitals Chapter 10 Copyright C The Mcgraw Hill Companies Inc Permission Required Ppt Download
If you are looking for Papa Francisco Frases you've arrived at the perfect location. We ve got 104 graphics about papa francisco frases including images, photos, pictures, backgrounds, and more. In these page, we additionally provide variety of images out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, translucent, etc.
Treat the double bonds as single bonds and see that it has 2 bonding pairs and one lone pair.

Papa francisco frases. The s in so2 has two double bonds one set of double bonds to each o and it has a lone pair. If it is linear then it must be like carbon dioxide. Voiceover in the previous video we looked at the dot structure for sulfur dioxide and i drew out two resonance structures.
According to common sense it is some where between linear and trigonal. What is the molecular shape of so2. The sulfate or sulphate see spelling differences ion is a polyatomic anion with the empirical formula so2 4.
Salts acid derivatives and peroxides of sulfate are widely used in industry. For thats the shape of so2. So the resonance structure on the left and the resonance structure on the right and some people disagreed with me and said thats not the dot structure for sulfur dioxide.
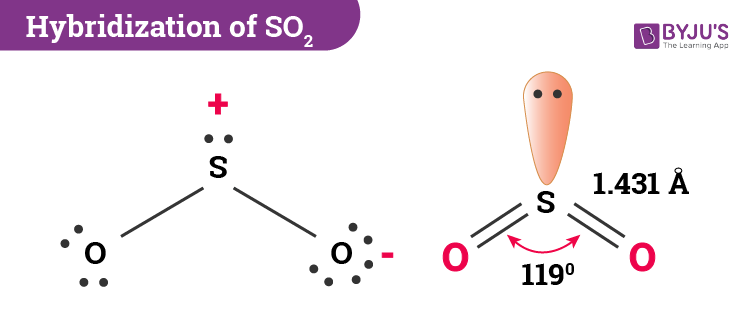
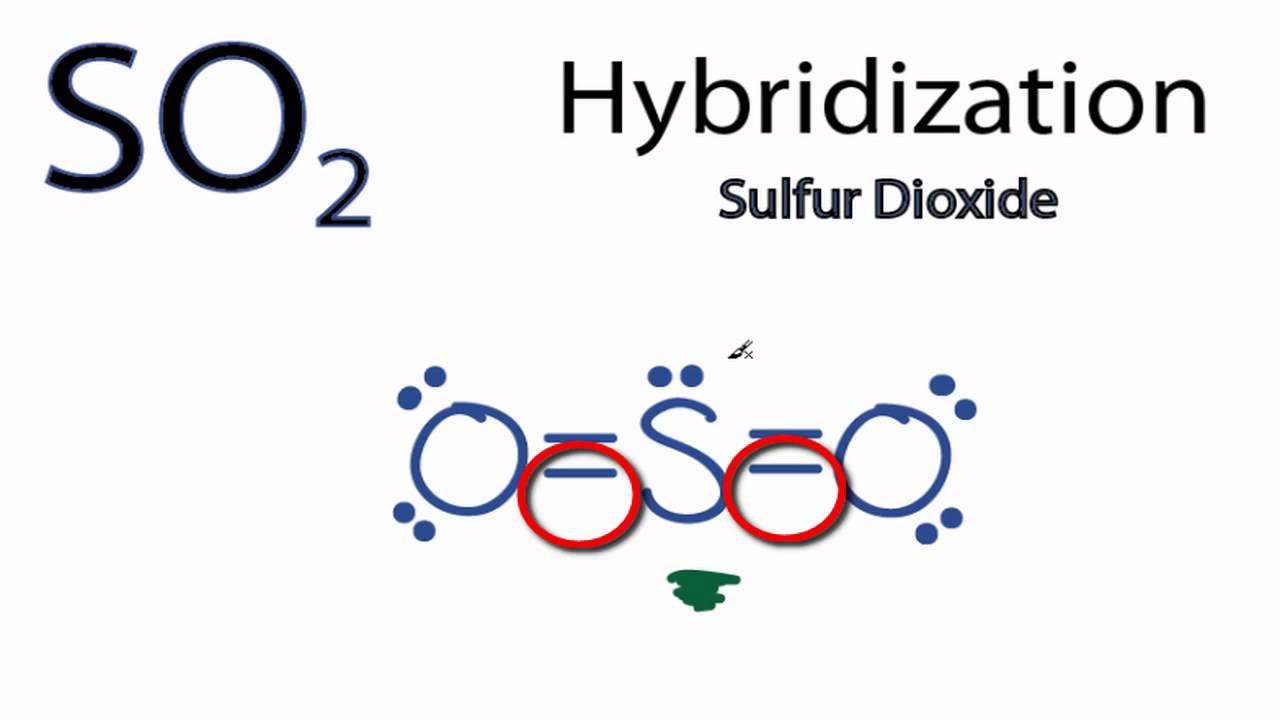
A valence bond theory approach considering just s and p orbitals would describe the bonding in terms of resonance between two resonance structures. It has a bent molecular structure with 120 degrees between the two oxygen atoms. That means trigonal planar electron geometry and a bent molecular shape.
Sulfate is the spelling recommended by iupac but sulphate is used in british english. Three atoms these are the only options. If you dont want to call it bent call it v shaped.
The molecular geometry of so2 has a bent shape which means the top has less electronegativity and the bottom placed atoms of oxygen have more of it. That makes three domains with electrons. So 2 is a bent molecule with c 2v symmetry point group.
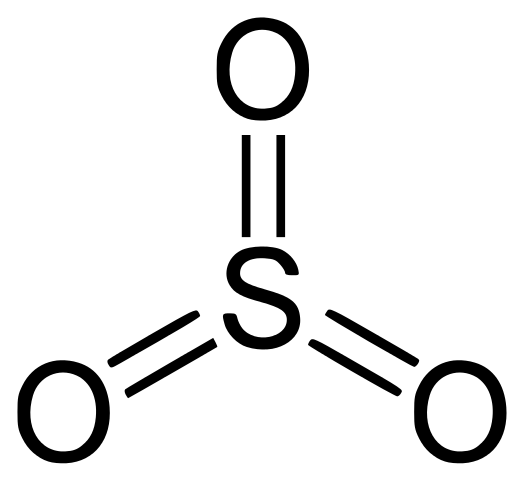
Sulfur dioxide or so2 contains 2 oxygen atoms double bonded to a sulfur atom.
More From Papa Francisco Frases
- Kapil Dev Son
- Manchester City
- Soal Caption Kelas 12 Dan Jawabannya
- Antwnhs Kafetzopoylos
- Upscaling Image
Incoming Search Terms:
- Why Is The Bond Angle Of Sulphur Dioxide Close To 120 Not 90 Chemistry Stack Exchange Upscaling Image,
- Which Of The Following Statements Is Not Correct For So2 Gas Upscaling Image,
- Covalent Bonding Shapes Valence Sheell Electron Pair Repulsion Ppt Video Online Download Upscaling Image,
- Chemistry Net Covalent Lewis Structure So2 Simple Procedure For Dot Structures 53 Upscaling Image,
- Upscaling Image,
- So2 Sulfur Dioxide Molecular Geometry Lewis Structure Geometry Of Molecules Upscaling Image,