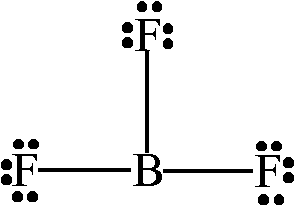Molecular Shape Of Bf3, Bf3 Molecular Shape
Molecular shape of bf3 Indeed lately has been sought by users around us, maybe one of you personally. People now are accustomed to using the net in gadgets to view image and video data for inspiration, and according to the name of the article I will talk about about Molecular Shape Of Bf3.
- What Is The Molecular Shape Of Bf3 Youtube
- Solved Choose A Lewis Structure For Bf3 Chegg Com
- Diagram Lewis Diagram Bf3 Full Version Hd Quality Diagram Bf3 Typewiringsubs Bbalpes Fr
- Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsxpzfenarkj07bdrzrfcvkd5ezslsaqjh2cgcjfb8 Usqp Cau
- Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity
- Bf3 Lewis Structure Shape Chilangomadrid Com
Find, Read, And Discover Molecular Shape Of Bf3, Such Us:
- Group Quiz Chemistry 301
- Valence Shell Electron Pair Repulsion Theory Vsepr Chemistry Study Material Emedicalprep Com Emedicalprep
- The Molecular Structure Of Bf 3 In The Gas Phase Refs 1 3 A Download Scientific Diagram
- Molecular Shapes Calango Free Online Courses
- According To The Vsepr Theory Which Shape Is Possible For A Molecule With The Molecular Formula Of Ab 3 Where The Number Of Total Electron Groups Is Unstated Socratic
If you re searching for 1956 Oktober 23 Forradalom Kepek you've come to the right place. We ve got 104 graphics about 1956 oktober 23 forradalom kepek including pictures, pictures, photos, wallpapers, and more. In these webpage, we also provide number of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, black and white, transparent, etc.

Valence Shell Electron Pair Repulsion Theory Vsepr Chemistry Study Material Emedicalprep Com Emedicalprep 1956 Oktober 23 Forradalom Kepek
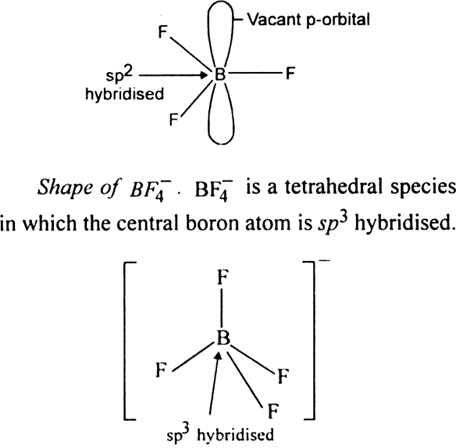
It is a useful lewis acid and a versatile building block for other boron compounds.

1956 oktober 23 forradalom kepek. This pungent colourless toxic gas forms white fumes in moist air. It is toxic by inhalation. Bf3 is a molecule in the trigonal planar shape.
It is soluble in water and slowly hydrolyzed by cold water to give off hydrofluoric acid a corrosive materialits vapors are heavier than air. The valency of b boron is 3 and of f fluorine is 7 thus the lewis structure of bf3 can be drawn as shown in the figure. Bf 3 molecular geometry and bond angles.
Normally boron forms monomeric covalent halides which have a planar triangular geometry. Prolonged exposure of the containers to fire or heat may result in their violent rupturing and rocketing. Boron trifluoride is a colorless gas with a pungent odor.
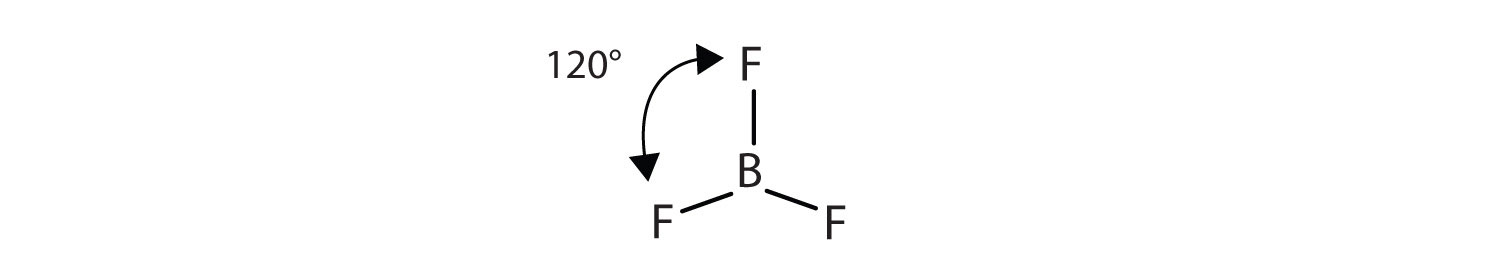
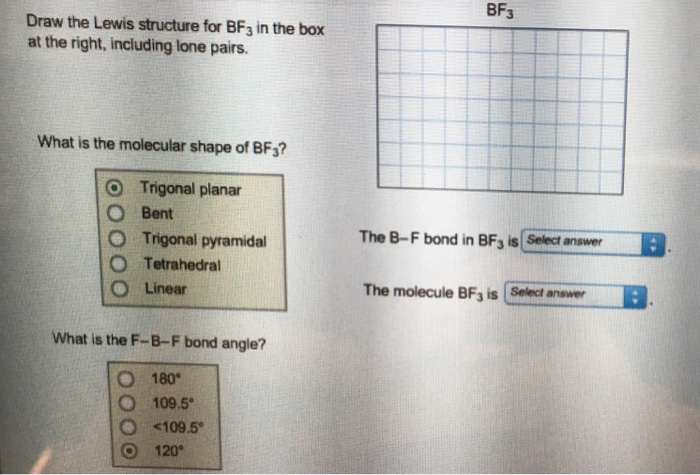
Its like peripheral atoms all in one plane as all three of them are similar with the 1200 bond angles on each that makes them an equilateral. Ax 3 has trigonal planar shape. It has a central boron atom that is surrounded on three sides by three fluorine atoms.
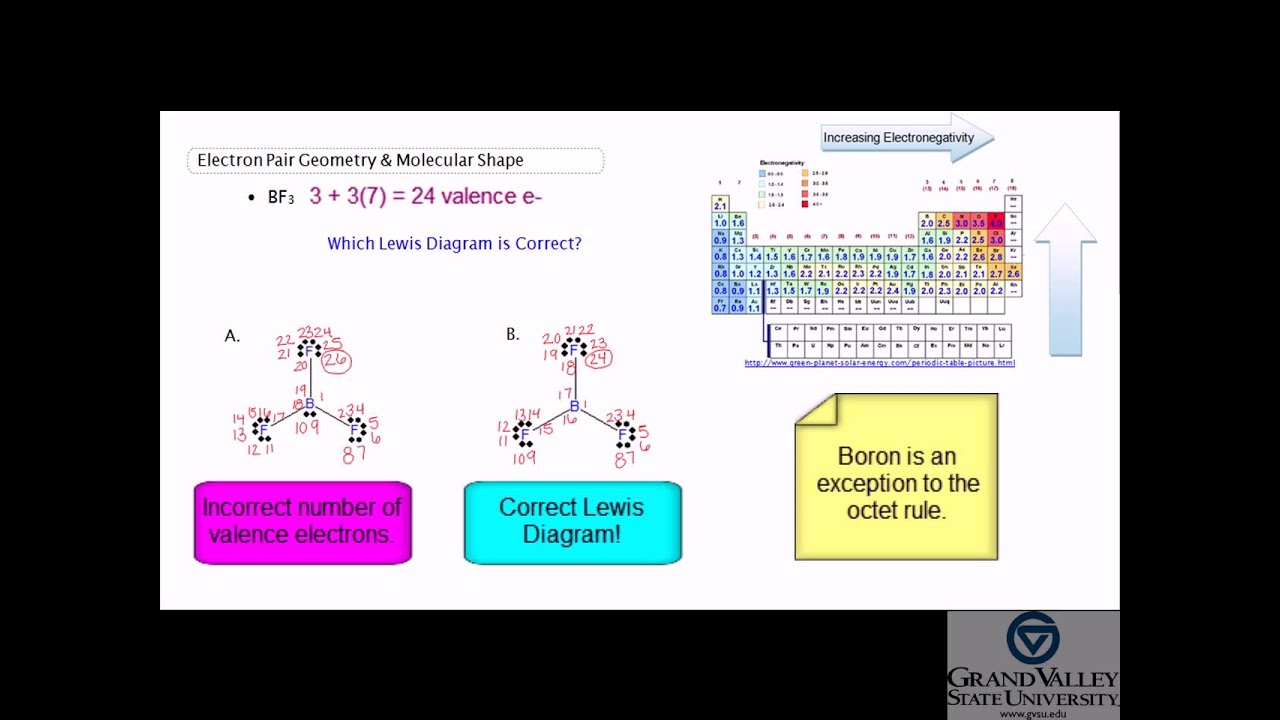
5 and 3 atoms of f fluorine atomic no. Molecular geometry of bf3. Molecular structure of bf3 boron trifluoride the bf3 boron trifluoride molecule has 1 atom of b boron atomic no.
Bf3 is a molecule in the trigonal planar shape. Boron trifluoride is the inorganic compound with the formula bf 3. Although the electron groups are oriented in the shape of a tetrahedron from a molecular geometry perspective the shape of nh 3 is trigonal pyramidal.
All the bonds in bf 3 are sigma bonds. Nh 3 is an example of a molecule whose central atom has four electron groups but only three of them are bonded to surrounding atoms. Use vsepr table to find the shape.
Bf 3 molecule is formed by bonding between three sp 2 orbitals of b and p of 3 f atoms. Download a copy of vsepr shapes table here bond angle in bf 3 bond angle of f b f covalent bond in this molecule is 180othe representation is shown below. The geometry of a molecule of bf 3 is trigonal planar.
The three hybridized sp 2 orbitals are usually arranged in a triangular shape. The geometry of molecule of bf3 is trigonal planar with the reference of chemistry trigonal planar is a model with three atoms around one atom in the middle.
More From 1956 Oktober 23 Forradalom Kepek
- 2019 Daycare Meal Rate Irs
- Cuando Es El Dia Del Medico
- Leggett Platt Split King Adjustable Bed Parts
- Personagens Dolivro O Homem Invisivel
- Ordinanza De Luca Oggi
Incoming Search Terms:
- Valence Shell Electron Pair Repulsion Theory Vsepr Chemistry Study Material Emedicalprep Com Emedicalprep Ordinanza De Luca Oggi,
- Solved Using Vsepr Theory Determine The Electron Group G Chegg Com Ordinanza De Luca Oggi,
- Molecular Shapes Ordinanza De Luca Oggi,
- The Shapes Of Molecules Ordinanza De Luca Oggi,
- Molecular Shapes Ordinanza De Luca Oggi,
- Which Of The Following Molecule Has A Bent Molecular Shape B Bf3 E Hcn D Nh3 Homeworklib Ordinanza De Luca Oggi,